This article first appeared in CHRISTIAN RESEARCH JOURNAL, volume 35, number 01 (2012). The full text of this article in PDF format can be obtained by clicking here. For further information or to subscribe to the CHRISTIAN RESEARCH JOURNAL go to: http://www.equip.org/christian-research-journal/
No one has ever accused Richard Dawkins of being bashful. On the contrary, Dawkins’s outspoken claims about the power of natural selection to explain the complexities of organisms have earned him a large following. “The theory of natural selection,” Dawkins states, “provides a mechanistic, causal account of how living things came to look as if they had been designed for a purpose.”1
What few of Dawkins’s followers realize, however, is how rarely Dawkins has used the theory of natural selection to explain the origin of anything. Now, one might say that Dawkins is a “big picture” guy; the details are the business of biologists in the laboratories.
But what if they don’t use natural selection either? What if natural selection, far from explaining the origin of biological design, in fact presupposes it?
BOLD CLAIMS, MISSING EVIDENCE
This article examines why natural selection, although a real process, does not live up to the claims made on its behalf by Dawkins and other New Atheists. To understand the disconnect between process and claim, we should focus on an aspect of biology where natural selection should be able to show its explanatory prowess: the origin of animal development.
This case study may be more biology than you ever expected to read in the pages of this journal. But, by the end of this article, you should be able to grasp fully the challenge that real biology makes to the unsupported assertions of atheists, who steal the good name of science.
THE PUZZLE OF ANIMAL DEVELOPMENT
Most people know intuitively that when they see the birth of a child they are witnessing something utterly remarkable, for which the word miracle is fitting. How could this baby have come to be, when nine months earlier, there was only a single cell, the fertilized egg? What many miss, however, is how this miracle extends to every animal on Earth, from the tiniest worm to the largest whale.
My colleague Ann Gauger and I have compared development to what we call a magical bridge, a structure that would be right at home in any Indiana Jones movie.2 Imagine a bridge (see figure 1) spanning a deep gorge. As long as one continues to walk across it, the bridge will be there beneath one’s feet. If one stops, however, or turns in the wrong direction, the bridge instantly disappears.
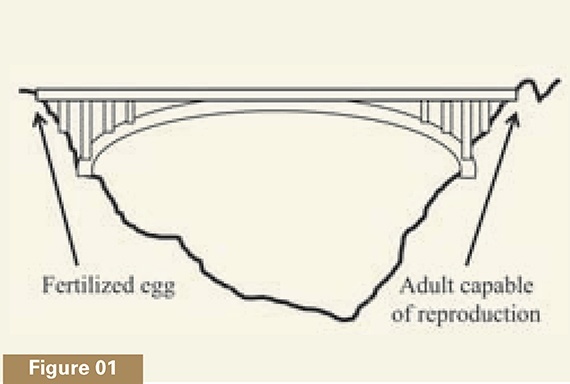
This “magical bridge” aspect to animal development is well known to biologists. In most animal species, development starts with a single cell, the fertilized egg. Once the egg divides into its daughter cells, becoming an embryo, the organism starts across its own bridge, where success means reaching the far side, namely, the ability to reproduce. Everything about development is directed to reaching this endpoint, and the entire pathway is needed. An embryo whose development is arrested midway, for instance, will die. Development is thus the paradigmatic teleological process in nature—where the telos (Greek for “end” or “goal”) is a reproductively capable adult organism (see Figure 2a). Each stage in development enables the next stage to occur, like the series of steps in a Google Maps printout of driving directions.
If one reflects on the nature of development for a moment, in relation to natural selection, a problem arises. How could a physical process with no foresight (namely, natural selection) construct a multistep pathway that seems to require foresight?
Let’s take a closer look at the logic of natural selection itself.
WHAT DOES NATURAL SELECTION REQUIRE?
Natural selection is a three-step process, with three jointly necessary and sufficient conditions:3 (1) Variation in some trait p; (2) Selection in relation to trait p: organisms with the trait must leave more offspring than those without it; (3) Heritability of the trait p: parents must be able to transmit the trait to their offspring.
If these conditions are satisfied, natural selection will occur: the frequency of p will change. But if any of these conditions is absent, natural selection cannot happen.
Another aspect of natural selection will be just as critical to understanding why development challenges natural selection. Compare Figure 2a, a developmental pathway, to 2b, which depicts a single-celled eukaryote (an organism with a nucleus), similar to the group thought to lie at the evolutionary root of the origin of the animals. Because natural selection lacks intelligence, “life never evolves with foresight,” as evolutionary biologist Andrei Rodin and colleagues explain: “Natural selection works strictly ‘in the present moment,’ right here and right now, just like a first-aid ambulance— lacking the foresight of potential future advantages…. Therefore, just as with any case of step-by-step evolution towards a more complex system, there should be an evolutionary rationale behind each intermediate step.”4
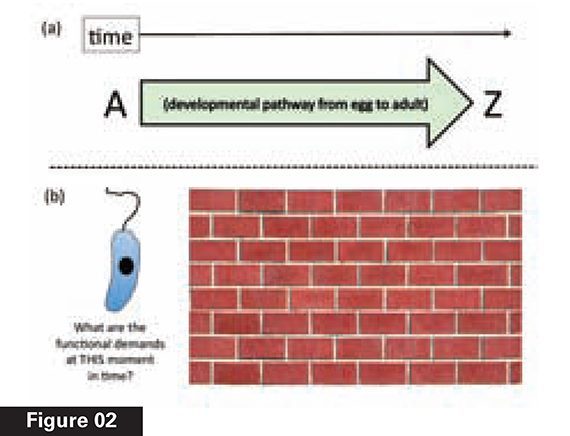
This lack of foresight is depicted metaphorically in Figure 2b as a brick wall. To be amplified by natural selection, any variation must display its selective advantage immediately— not in the future. Natural selection cannot “see” through the brick wall, in other words, beyond the immediate functional role of any variation.
This entails that if some feature requires multiple independent stages, natural selection will be able to construct it only if each newly arising stage is heritable (condition 3 of selection) and provides some functional advantage (condition 2). Put another way, natural selection will be unable in principle to construct multistep processes such as development, if the steps individually provide no advantage, and are not heritable, until the whole process is complete. If one needs to cross the magical bridge, walking out part way will not do.
EVOLVING A TOY ANIMAL FROM SCRATCH
To grasp the problem, we need a simplified model. Even the tiniest real animal possesses hundreds of specialized cells, far more than we can handle within this article. The principles that our model illuminates, however, apply to the much more complicated features of any real animal.
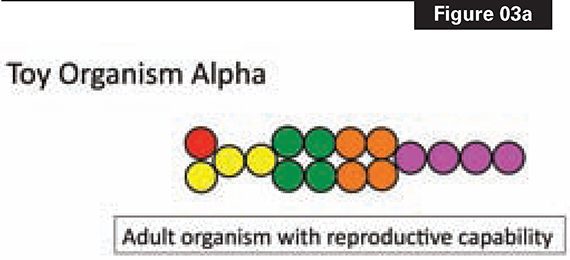
Figure 3a depicts a toy animal (Alpha) with sixteen cells of five types. Figure 3b shows how Alpha is constructed. A single cell gives rise, through four rounds of division, to the sixteen cells of the “adult.” The “adult” stage of Alpha enables reproduction and heredity to happen.
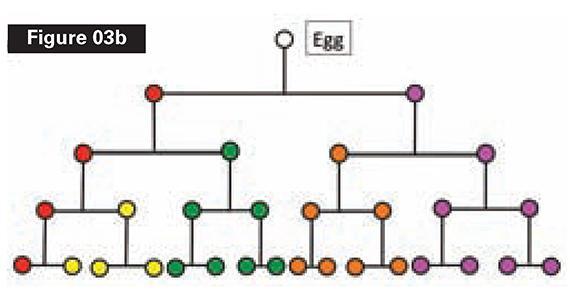
Could natural selection have constructed the pathway in 3b? Remember, in our scenario, Alpha and its developmental pathway do not yet exist. We are going to use natural selection to try to construct the pathway, starting from a single-celled ancestor.
Let’s look at just the first two rounds of cell division, which yield four cell types (see Figure 4). The starting point will be a single cell—Cell Zero—which divides.
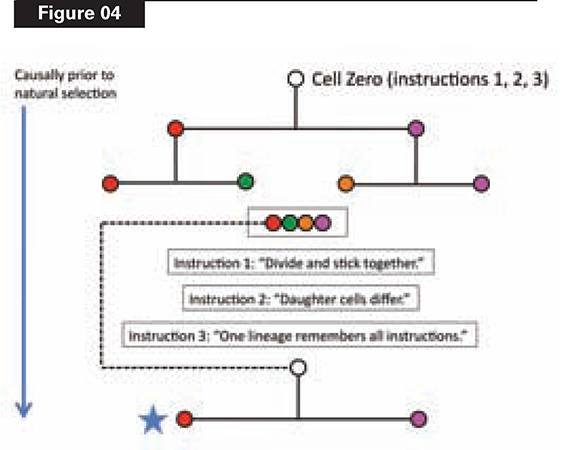
But this won’t work for building the lineage, unless the daughter cells remain physically connected. So Cell Zero needs the instruction, (1), “divide and stick together.” Clearly, this instruction must be in place before Cell Zero divides.
Now we have two daughter cells. They are identical, however. To produce the pathway that builds Alpha, we need the daughter cells to be different. Thus, unless we provide another instruction to Cell Zero, we’ll have a mass of identical cells. Cell Zero therefore needs another instruction, (2), “daughter cells differ,” which again must be there before Zero divides. (See Figure 4.)
Then the two daughter cells divide, yielding four cells of four different types. Have we successfully constructed even this starting pathway (never mind the other twelve cells) via natural selection?
No. Natural selection cannot operate, because one of its necessary conditions, heredity, has not been satisfied. This organism (of four differentiated cells) must leave offspring, to which it transmits its distinctive variations. Condition (3) of the process of natural selection, heredity, requires that variations arising in a parent be transmitted to offspring. This means that at least one of four cells must retain the instructions for the whole lineage, if the cycle is going to repeat in the next generation.
Cell Zero therefore needs yet another instruction, (3), before it divides: “At least one cell line must maintain the whole instruction set, and start another cycle.” This is the functional reason animals need a germ line (i.e., cells that produce gametes): those cells carry the instructions for the whole organism.
NATURAL SELECTION REQUIRES COMPLEXITY IT CANNOT CONSTRUCT
What is the bottom line of our toy story? Natural selection cannot begin to operate until reproductive capability arises, and reproduction occurs. The blue star in Figure 4 shows the point at which this necessary condition is satisfied.
Everything upstream of the blue star, therefore, must have been constructed by some cause other than natural selection. Figure 4 shows that we need to “build an egg from scratch,” namely, Cell Zero, by loading it with the functionally necessary instructions required before it divides. Reproduction of the whole lineage is causally downstream of all this egg-building work. Whatever instructions need to be present in Cell Zero, therefore, must be there before natural selection could operate.
If you doubt this, construct your own toy developmental pathway. Figure 4 depicts a decision tree, and the instructions in the starting node (Cell Zero) to specify that tree and to cycle it through successive generations. Try to find the minimal instruction set required in the starting point to generate the endpoints, and to repeat the process. Remember that, if you want the whole lineage to repeat (“reproduce”), any decision affecting any endpoint must be front-loaded into the start.
The problem we have been examining is well known to biologists who study the origin of animal development. One such biologist, Lewis Wolpert of University College, London, calls the mystery “sadly neglected.” “Since embryonic development requires the formation of a multicellular organism from a single cell,” he observed, “the origin of the egg is a central and sadly neglected problem.”5 The puzzle is the origin of heritable differentiation: “The key to all development is the generation of differences between the cells, that is, making them non-equivalent [see Figures 3a and b, above]. Only if the cells are different can the organism be patterned so that there are organized changes in shape, and cells at specific sites differentiate into different cell types. How could this have evolved?”6
One might think that only a mass of cells is needed—a colony of single-celled eukaryotes, perhaps, which happen to adhere—and that differentiation could evolve slowly by specialization somehow occurring within this mass. Some cells become “body,” while others enable reproduction, forming “the germ line.”
But what would coordinate gene expression among all the members of the colony, such that only one cell lineage will evolve to carry the complete instruction set required to specify the form of the whole? How are mutations—occurring in all individual cells of the colony—transmitted to the next generation? If individual cells continue to reproduce via normal cell division, notes Wolpert, “cell lineages [will be] mutating in all sorts of directions in genetic space.”7 Given such genetic chaos, he argues, “we consider it practically impossible” for the collection of cells to “yet retain the ability to evolve into viable new forms.”8
To evolve the developmental pathway leading to the distinctive form of any animal, Wolpert contends, requires mutations affecting a single key cell—the egg. Mutations affecting the cells of the body, by contrast, cannot be coordinated: “There is no way that the genes in the huge number of cells…can change at the same time, and mutations in individual cells mean that they no longer share the behavioural rules of the majority. It is only through a coherent developmental programme, with all cells possessing the same genes, that organisms can evolve, and this requires an egg.”9
And an egg requires engineering, which natural selection cannot provide.
NO FORESIGHT? NO SOLUTION!
If one looks in Richard Dawkins’s voluminous writings for the solution to this puzzle, one won’t find it. Nor will one find it in the technical literature of biology. “How cell types of multicellular organisms came to be differentiated,” notes evolutionary biologist Carl Schlichting, “is still an open issue.”10
The puzzle cannot be solved within any evolutionary framework operating without foresight. The pathway from egg to adult, in all animal species that undergo such a process, requires a cause that could have seen the distant target—a reproductively capable adult—from the start.
And, in our universe, what is the only cause with foresight? Intelligence: a mind—like yours, only vastly more powerful.11
Paul Nelson, Ph.D. is an adjunct professor in the MA Program in Science and Religion at Biola University, and a Fellow of the Discovery Institute, specializing in developmental biology and evolution.
NOTES
- Richard Dawkins, “Replicator and Vehicles,” in Kings College Sociobiology Group, eds., Current Problems in Sociobiology (Cambridge: Cambridge Univ. Press, 1982), 45.
- Paul Nelson and Ann Gauger, “Stranger Than Fiction: The Riddle of Metamorphosis,” in Metamorphosis: The Case for Intelligent Design in a Chrysalis, ed. D. Klinghoffer (Seattle: Discovery Institute Press, 2011).
- John Endler, Natural Selection in the Wild (Princeton: Princeton University Press, 1986).
- Andrei S. Rodin, Eörs Szathmáry, and Sergei N. Rodin, “On the Origin of the Genetic Code and tRNA before Translation,” Biology Direct 6 (2011): 14.
- Wolpert, “The Evolutionary Origin of Development: Cycles, Patterning, Privilege and Continuity,” in The Evolution of Developmental Mechanisms, ed. M. Akam et al. (Development 1994 Supplement; Cambridge: The Company of Biologists Ltd.), 79–84.
- Ibid., 80.
- Wolpert, “Evolution and the Egg,” Nature 420 (2002): 745.
- Ibid.
- Ibid.
- Carl Schlichting, “Origins of Differentiation via Phenotypic Plasticity,” Evolution and Development 5 (2003): 98–105.
- Portions of this article, and figures 1–4, have been reproduced with permission from the series on ontogenetic depth published in April 2011 by the Discovery Institute (at www.evolutionnews.org). Figure 1 is reproduced with permission from the e-book Metamorphosis: The Case for Intelligent Design in a Chrysalis (Discovery Institute Press, 2011).